hybridization of xef4|xef4 vsepr shape : Clark Today in this video, we are going to learn the hybridization of the XeF4 molecule. It is a chemical formula for Xenon Tetrafluoride. To understand the . PinayFlix TV; Pinay Porn Videos; Contact Us; Latest videos. Latest videos Longest videos Random videos. HD 04:26. Pinay na mahilig mang thirst trap pumatong kay Ralph. HD 05:11. Nakatikim ng jumbo hatdog si petite pinay. HD 03:22. Ayaw lunukin medyo malansa kasi. HD 03:32. Kinawawa ni Armani ang aking pussy. HD 06:58. Pinadede si damulag.
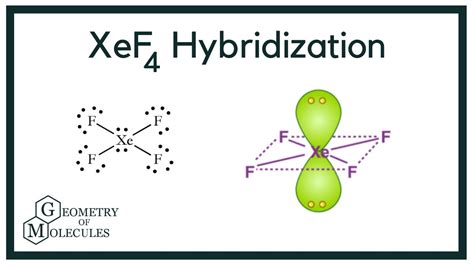
hybridization of xef4,Learn about the hybridization of XeF4, XeOF4, XeF5 and IF5, and their molecular geometries and structures. Find out how the central atom Xe uses its s, p and d orbitals to form sp2d2, sp3d2 or sp3d3 hybrid orbitals. Learn how XeF4 is formed by the migration of two electrons from 5p to 5d orbitals of Xe, resulting in sp 3 d 2 hybridization. Find out the molecular geometry, bond . Today in this video, we are going to learn the hybridization of the XeF4 molecule. It is a chemical formula for Xenon Tetrafluoride. To understand the .Learn how xenon tetrafluoride (XeF4) forms a square planar structure with four single bonds and two lone pairs. Find out the hybridization of XeF4 and the orbi. Learn about the sp3d2 hybridization of Xenon in Xef4, a binary compound of Xenon and Fluorine. Find out how the Lewis structure, bond angles and nonbonding pairs of electrons determine . Xe is d^2sp^3 hybridised, and F is sp^3 hybridised. This is the Lewis structure of XeF_4: The steric number, or the number of atoms and lone pairs, of Xe is . Xenon tetrafluoride is a chemical compound with chemical formula \(\ce{XeF4}\). It was the first discovered binary compound of a noble gas. It is produced .
#XeF4_hybridization #XeF4_lewis_structure #sp3d2_hybridizationThe structure/shape/molecular geometry of Xenon tetrafluoride - XeF4 sp3d2 hybridization .Learn how to draw the Lewis structure, molecular and electron geometry of XeF4 based on the VSEPR theory, the steric number and the sp3d2 hybridization. See examples and explanations of the octahedral and . An explanation of the molecular geometry for the XeF4 (Xenon tetrafluroide) including a description of the XeF4 bond angles. The electron geometry for the Xenon .Hybridization in Xenon Tetrafluoride: One might find it strange that a noble/inert gas can make bonds, but there are a few of the noble gases that can do this because of atomic orbital hybridization. Atomic orbital hybridization is where s, p, and sometimes d orbitals blend together (hybridize) to form new orbitals that explain the shapes and . An explanation of the molecular geometry for the XeF4 (Xenon tetrafluroide) including a description of the XeF4 bond angles. The electron geometry for the Xe.

Conclusion. The Lewis structure for XeOF4. The molecular geometry of the XeOF4 molecule is square pyramidal. The hybridization state for the XeOF4 molecule is sp3d2. XeOF4 is a polar molecule. Happy learning!! Xenon Oxytetrafluoride is a colorless inorganic compound. Similar to other oxides of Xenon it is also very unstable and highly . The hybridization of XeF4 in its Lewis structure is sp3d2. This is due to the presence of six electron domains – four bonding pairs and two lone pairs. What is the Lewis structure for XeF4? The Lewis structure for XeF4 consists of Xenon (Xe) in the center surrounded by four Fluorine (F) atoms. Xenon also has two lone pairs of electrons.Click here:point_up_2:to get an answer to your question :writing_hand:the correct geometry and hybridization for xef4 arehybridization of xef4 xef4 vsepr shape The shapes of the first five atomic orbitals: 1s, 2s, 2p x, 2p y, and 2p z. The colors denote the sign of the wave function. Orbital hybridization involves making linear combinations of the atomic orbitals that are solutions to the Schrödinger equation.xef4 vsepr shape Xenon tetrafluoride (XeF4) Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge, hybridization. XeF 4 is the chemical formula for xenon tetrafluoride, the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon (Xe) with fluorine (F 2) and exists as a colorless .The hybridization in xenon tetrafluoride occurs in the central atom, Xenon (Xe). When we look at Xe’s valence shell, we can see six ions in the 5p orbital and a pair of electrons in the 5s earth orbit. If we look at the 5th shell, we can see no electrons in the d and f orbitals. In creating XeF4, the 5p planetary electron pairs’ pairs move . Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s and 2p z atomic orbitals, for example, we produce two new orbitals with major and minor lobes oriented along the z .Symmetry, Spectroscopy, and the Molecular Structure of XeF4 The infrared spectrum of XeF4 has absorptions at 161, 291, and 586 cm-1 (two bends, one stretch), while the Raman spectrum has peaks at 218, 524, and 554 cm-1 (one bend, two stretches). Is its molecular structure tetrahedral or square planar? References: J. Am. Chem. Soc.
Click here:point_up_2:to get an answer to your question :writing_hand:hybridization and structure of xef4 isIn order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence . Determine the geometry of the molecule using the strategy in Example 10.7.1 10.7. 1. From the valence electron configuration of the central atom and the number of electron pairs, determine the .
Hybridization. Hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. This is part of the valence bond theory and helps explain bonds formed, the length of bonds, and bond energies; however, this does not explain molecular geometry very well. sp An example of this is acetylene (C 2 H 2 ).Step 1. Hybridization: −. The phenomenon of intermixing of orbitals is known as hybridization. View the full answer Answer. Unlock. Previous question Next question. LEWIS STRUCTURE -HYBRIDIZATION

$$ XeF_4: sp^3 d^2 $$ hybridization. Six electron pairs around central atom four bond pairs, two lone pairs so square planar shape. $$ OSF^4: sp^3d $$ hybridizedsp 2 hybridization. sp 2 hybridization can explain the trigonal planar structure of molecules. In it, the 2s orbitals and two of the 2p orbitals hybridize to form three sp orbitals, each consisting of 67% p and 33% s character. The frontal lobes align themselves in the trigonal planar structure, pointing to the corners of a triangle in order to minimize .
hybridization of xef4|xef4 vsepr shape
PH0 · xenon tetrafluoride hybridization
PH1 · xef4 vsepr shape
PH2 · xef4 lone pairs
PH3 · types of hybridization in chemistry
PH4 · types of hybridization
PH5 · sigma bonds in xef4
PH6 · hybridization of nh3
PH7 · ch4 hybridization of central atom
PH8 · Iba pa